Medical Evidence
Glucosamine Basic Science Studies
Chondroitin Basic Science Studies
Glucosamine Clinical (Human) Studies
Chondroitin Clinical (Human) Studies
Combined Clinical (Human) Studies
Review Articles on Glucosamine
Review Articles on Chondroitin
Home Page
News
Supplements
Resources
Products
Books
Q & A
About Dr. Theo
 
|
Celebrex® fails in GAIT (Glucosamine/Chondroitin Arthritis Intervention Trial) - Invalidates the negative results in the study
The drug, Celebrex was used as an active control drug in GAIT. Active control drugs are used to monitor the quality of the study. If the active control drug fails to perform like it has in other, previous studies, then all of the results deemed to be negative should be considered invalid or void.
Much of the media seemed to have only focused on one finding in GAIT without looking at the performance of the active control drug to see if this study is even relevant. The active comparator drug must show a positive effect in all of these measures or something was wrong about the study design or implementation. The five measures of importance in this type of study are:
1- Statistical significance of each treatment compared to placebo
2- Statistical significance compared to other treatments in the study
3- Clinically meaningful findings
4- Odds of being a responder to the active comparator drug
5- Effect sizes
As you'll see below, Celebrex only partially passes #1, but fails #2-5. GAIT is a thus a voided study. This easily fool the media, public and even some physicians who are not knowledgeable about statistics and research design.
Statistical significance compared to placebo
Below is the chart showing all of the GAIT results that were significantly better than the placebo group (which had a 60% response itself in the primary outcome, thus washing out the data.)
The supplements were (statistically significantly) effective in six categories, two more than the prescription drug Celebrex.
It is not plausible that Celebrex failed to reach significance in only 40/42 possible outcome measures. This is completely contradictory to dozens of other studies involving Celebrex. Since the data was washed out for Celebrex, it was also washed out for glucosamine and chondroitin.

Statistical significance compared to other treatments in the study
In GAIT, the investigators did not directly compare the treatments. Treatments were only compared individually to placebo. Had a direct comparison been made, there would be no difference in effect between the supplement groups and Celebrex. If the difference between the glucosamine group (65% of the subjects responded compared to 60% for the placebo group) was not considered statistically significant, then the difference between the glucosamine + chondroitin group (67% of the subjects responded compared to 70% for the Celebrex group) is certainly not significantly different. In the real world, since Celebrex is about 3-5 times as expensive for a daily dose, requires co-medication use in some cases, and increases the risk of high blood pressure, stroke and heart attack, the supplements should be first line therapy. Celebrex fails to beat the supplements.
Clinically Meaningful Results
Before the study began, the GAIT investigators determined that a "clinically meaningful treatment effect of 15% or greater compared to placebo was needed. Celebrex failed to reach this goal. Only the combination of glucosamine + chondroitin in those who has the most pain reached this goal. The supplement combination outperformed Celebrex in this outcome.
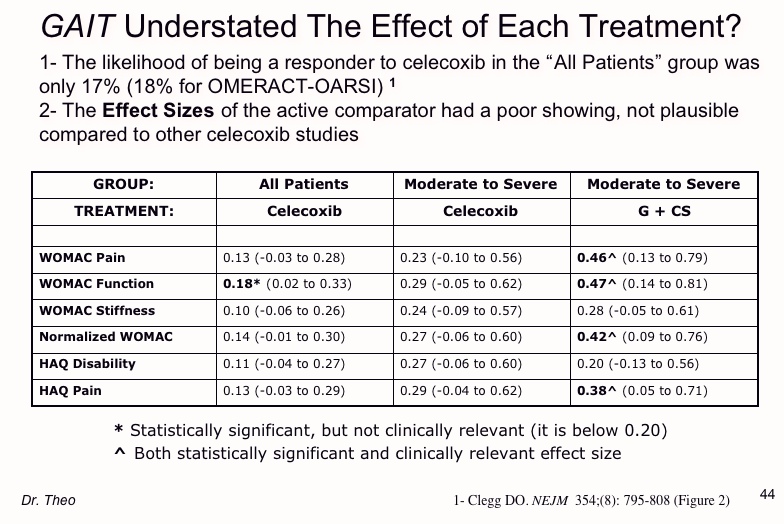
Odds of being a responder to the active comparator drug & Effect Sizes
If a subject gets a treatment in a study such as GAIT, one can determine the chance that they responded to a particular treatment. This is important and has real-world implications. Suppose a doctor treats 100 patients with a drug for osteoarthritis. If there's an 80% likelihood that those who take the drug get relief, and only 1% develop adverse reactions, then the benefit/risk ratio of this treatment may be favorable.
In GAIT, the odds of getting relieve from Celebrex was only 17-18%. This is much too low to risk the problems from Celebrex (fatal bleeding, stroke, heart attack, kidney and liver damage, high blood pressure, and others).
In many ways, effect sizes are more important than statistical significance compared to placebo.
Effect sizes are classified as low (0.2), medium (0.5) or high (0.8). Effect sizes below 0.2 are not meaningful. An Effect size is statistically significant if its confidence interval is above zero.
In GAIT, only the combination of glucosamine + chondroitin in the
moderate/severe pain group had significant effect sizes. Celebrex failed to reach both goals. The supplement combination outperformed Celebrex in these outcomes.
Conclusion:
In GAIT, because the active drug failed most measures, any failures in the supplement groups are not valid- they may be falsely negative. The negative results from the supplements should never be used as evidence in anther study, such as a review article.
Despite the dramatic failure of the study design and implementation, the only treatment group that met all of the criteria for success was the cobination of glucosamine and chondroitin in the patients who had moderate to severe pain.
|

Learn about and purchase the book90% of people who follow The Arthritis Cure treatment program don't need anti-inflammatories (like Aleve, Celebrex or Advil).
Dr. Theo warned people that these drugs, used first... read more
|
|






